- Home
- Science
- Science News
- Researchers Use Aluminium to Give Lithium Ion Batteries a Boost
Researchers Use Aluminium to Give Lithium-Ion Batteries a Boost
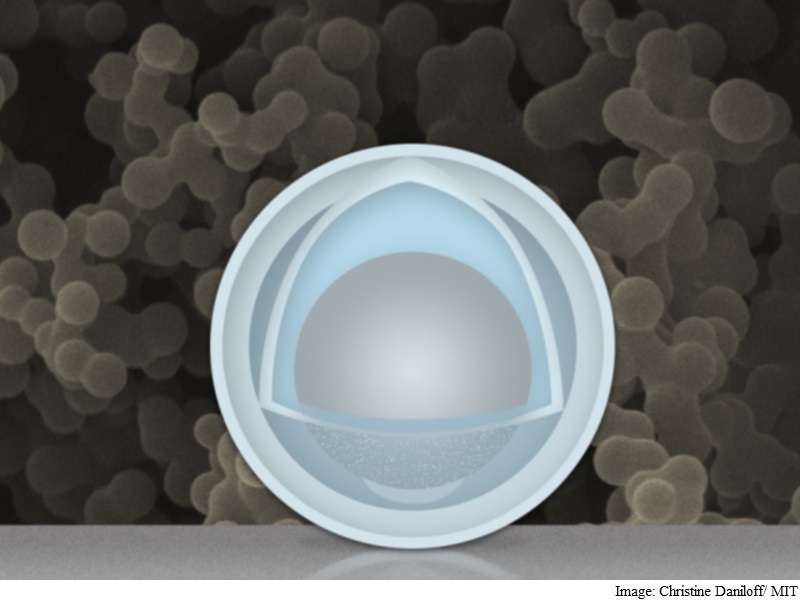
Team of researchers at MIT and Tsinghua University in China has found a novel way around that problem: creating an electrode made of nanoparticles with a solid shell, and a "yolk" inside that can change size again and again without affecting the shell. The innovation could drastically improve cycle life, the team says, and provide a dramatic boost in the battery's capacity and power.
Researchers at the Massachusetts Institute of Technology and Tsinghua University in Beijing found that the metal could expand and shrink freely by encasing the aluminium inside a shell. The battery has four times the capacity of current lithium-ion batteries and degrades less over time. It uses nanoparticles with a shell of titanium dioxide wrapped around aluminium, which acts as the battery's negative electrode.
The research overcomes previous problems experienced using aluminium in rechargeable lithium-ion batteries. The result is an electrode that gives more than three times the capacity of graphite (1.2 Ah/g) at a normal charging rate, Li says. At very fast charging rates (six minutes to full charge), the capacity is still 0.66 Ah/g after 500 cycles. The materials are inexpensive, and the manufacturing method could be simple and easily scalable, MIT professor Ju Li says.
The study was published in the journal Nature Communications. The research team included Sa Li, Yu Cheng Zhao, and Chang An Wang of Tsinghua University in Beijing and Junjie Niu, Kangpyo So, and Chao Wang of MIT. The work was supported by the National Science Foundation and the National Natural Science Foundation of China.
Written with inputs from PTI
For details of the latest launches and news from Samsung, Xiaomi, Realme, OnePlus, Oppo and other companies at the Mobile World Congress in Barcelona, visit our MWC 2026 hub.
Related Stories
- Samsung Galaxy Unpacked 2026
- iPhone 17 Pro Max
- ChatGPT
- iOS 26
- Laptop Under 50000
- Smartwatch Under 10000
- Apple Vision Pro
- Oneplus 12
- OnePlus Nord CE 3 Lite 5G
- iPhone 13
- Xiaomi 14 Pro
- Oppo Find N3
- Tecno Spark Go (2023)
- Realme V30
- Best Phones Under 25000
- Samsung Galaxy S24 Series
- Cryptocurrency
- iQoo 12
- Samsung Galaxy S24 Ultra
- Giottus
- Samsung Galaxy Z Flip 5
- Apple 'Scary Fast'
- Housefull 5
- GoPro Hero 12 Black Review
- Invincible Season 2
- JioGlass
- HD Ready TV
- Latest Mobile Phones
- Compare Phones
- Realme C83 5G
- Nothing Phone 4a Pro
- Infinix Note 60 Ultra
- Nothing Phone 4a
- Honor 600 Lite
- Nubia Neo 5 GT
- Realme Narzo Power 5G
- Vivo X300 FE
- MacBook Neo
- MacBook Pro 16-Inch (M5 Max, 2026)
- Tecno Megapad 2
- Apple iPad Air 13-Inch (2026) Wi-Fi + Cellular
- Tecno Watch GT 1S
- Huawei Watch GT Runner 2
- Xiaomi QLED TV X Pro 75
- Haier H5E Series
- Asus ROG Ally
- Nintendo Switch Lite
- Haier 1.6 Ton 5 Star Inverter Split AC (HSU19G-MZAID5BN-INV)
- Haier 1.6 Ton 5 Star Inverter Split AC (HSU19G-MZAIM5BN-INV)
















