- Home
- Science
- Science News
- New Material to Increase Solar Cell Efficiency
New Material to Increase Solar Cell Efficiency
To connect an existing silicon solar cell to a battery that splits the water may well be an efficient solution; but it is very expensive.
So, researchers were streamlining their search to a semi-conductor material that is able to both convert sunlight into an electrical charge and split water.
The team found gallium phosphide (GaP), a compound of gallium and phosphide, useful in this respect.
GaP has good electrical properties but the drawback is that it cannot easily absorb light when it is a large flat surface as used in GaP solar cells, said the study that appeared in Nature Communications.
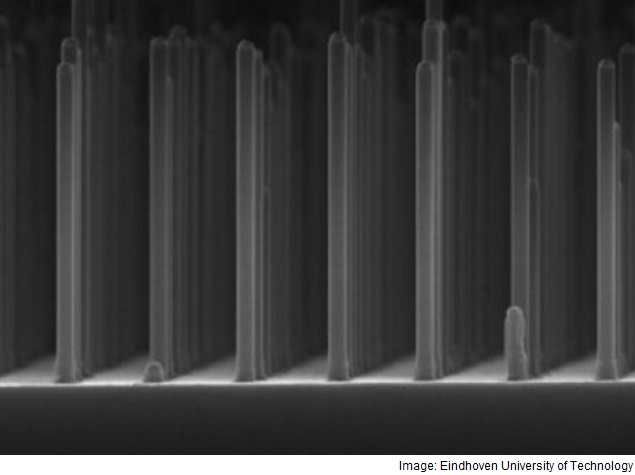
The researchers overcame this by making a grid of very small GaP nanowires, measuring five hundred nanometres (a millionth of a millimetre) long and ninety nanometres thick.
"That makes these kinds of cells potentially a great deal cheaper," said lead author Erik Bakkers from Eindhoven University of Technology, the Netherlands.
This immediately boosted the yield of hydrogen by a factor of ten to 2.9 percent.
"In short, for a solar fuel future, we cannot ignore gallium phosphide any longer," Bakkers added.
For the latest tech news and reviews, follow Gadgets 360 on X, Facebook, WhatsApp, Threads and Google News. For the latest videos on gadgets and tech, subscribe to our YouTube channel. If you want to know everything about top influencers, follow our in-house Who'sThat360 on Instagram and YouTube.
Related Stories
- Samsung Galaxy Unpacked 2025
- ChatGPT
- Redmi Note 14 Pro+
- iPhone 16
- Apple Vision Pro
- Oneplus 12
- OnePlus Nord CE 3 Lite 5G
- iPhone 13
- Xiaomi 14 Pro
- Oppo Find N3
- Tecno Spark Go (2023)
- Realme V30
- Best Phones Under 25000
- Samsung Galaxy S24 Series
- Cryptocurrency
- iQoo 12
- Samsung Galaxy S24 Ultra
- Giottus
- Samsung Galaxy Z Flip 5
- Apple 'Scary Fast'
- Housefull 5
- GoPro Hero 12 Black Review
- Invincible Season 2
- JioGlass
- HD Ready TV
- Laptop Under 50000
- Smartwatch Under 10000
- Latest Mobile Phones
- Compare Phones
- Moto G15 Power
- Moto G15
- Realme 14x 5G
- Poco M7 Pro 5G
- Poco C75 5G
- Vivo Y300 (China)
- HMD Arc
- Lava Blaze Duo 5G
- Asus Zenbook S 14
- MacBook Pro 16-inch (M4 Max, 2024)
- Honor Pad V9
- Tecno Megapad 11
- Redmi Watch 5
- Huawei Watch Ultimate Design
- Sony 65 Inches Ultra HD (4K) LED Smart TV (KD-65X74L)
- TCL 55 Inches Ultra HD (4K) LED Smart TV (55C61B)
- Sony PlayStation 5 Pro
- Sony PlayStation 5 Slim Digital Edition
- Blue Star 1.5 Ton 3 Star Inverter Split AC (IC318DNUHC)
- Blue Star 1.5 Ton 3 Star Inverter Split AC (IA318VKU)















